Authors
Srinivasan S, Manoj V
Received
3 March 2021
Accepted for publication
23 April 2021
Published
9 June 2021Volume 2021:15 Pages 2421—2435
DOI
https://doi.org/10.2147/OPTH.S294427
Checked for plagiarism
Yes
Review by
Single anonymous peer review
Peer reviewer comments
3
Editor who approved publication:
Dr Scott Fraser
Download Article
[PDF]
Sruthi Srinivasan, Venkiteshwar Manoj Clinical Development and Medical Affairs, Alcon Research, LLC, Johns Creek, GA, USACorrespondence: Sruthi SrinivasanClinical Development and Medical Affairs, Alcon Research, LLC, 11460 Johns Creek Parkway, Johns Creek, GA, 30097, USATel +1 678 415-5315Email
[email protected]
Abstract:
Dry eye disease (DED) is a multifactorial ocular condition characterized by a loss of homeostasis of the tear film resulting in ocular symptoms of discomfort, irritation, and visual disturbance, all of which significantly impact the patients’ social and occupational quality of life. While management of DED depends on the severity of symptoms and signs, use of artificial tear products (ATPs) that replace or supplement the deficient natural tear film is the mainstay treatment option. In this review, we present a decade of evidence on Systane Ultra
®
(polyethylene glycol [PEG]/propylene glycol [PG] with hydroxypropyl guar [HP guar]) in effectively managing DED. The active demulcents in Systane Ultra
®
—PEG, PG, along with HP guar gelling technology—provide optimal ocular surface protection and lubrication to heal damaged areas of the cornea caused by DED and, therefore, are recommended for patients with both aqueous and/or mucin layer deficiencies. Over the years, several clinical studies have shown that PEG/PG with HP guar provides long-lasting relief from dry eye and has often been chosen as a standard or comparator against other ATPs. Here, we describe the salient features of PEG/PG with HP guar—its constituents and their mechanisms of action. Furthermore, we summarize results from a systematic literature search that identified 23 relevant publications further emphasizing on the effectiveness and safety of PEG/PG with HP guar in alleviating the signs and symptoms of DED.
Keywords:
artificial tear products, dry eye disease, PEG/PG with HP guar
Dry Eye Disease: Prevalence, Definition, Burden, and Management
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a critical and significant public health issue affecting ~344 million people worldwide and more than 30 million in the United States alone [
https://www.tfosdewsreport.org/
, last accessed 10 February 2020]. The estimated prevalence of DED ranges from 5% to 34% in individuals over 50 years old and is more common in women; however, with increased use of electronic/media devices the risk of DED in the younger population is on the rise.
1
,
2
In 2017, the Tear Film & Ocular Surface Society’s Dry Eye Workshop II (TFOS DEWS II) report defined DED as a “multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface”. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.
3
,
4
Patients with DED may complain of ocular discomfort including redness, burning and stinging, ocular dryness, photophobia, foreign body sensation, grittiness, and visual disturbance, all of which significantly affect the quality of lives of patients (reporting up to 34% impairment in daily activities).
5
,
6
Etiologically, DED is classified into (i) aqueous-tear deficiency, characterized by lack of tear secretion from the lacrimal glands, and (ii) evaporative DED, involving excessive evaporative loss of tears due to meibomian gland deficiencies.
2
However, most DED (>80%) are mixed conditions characterized by both lacrimal and meibomian gland deficiencies.
7
With the increasing burden of DED and its impact on patients’ daily and social lives that worsens with age, it is important to manage this condition appropriately.
4
Depending on severity of the disease, the treatment and management of DED include patient education (about the condition, management, and prognosis); modifications in the environmental, dietary, and lifestyle-related factors; artificial tear substitutes; punctal plugs; lid warming and intranasal stimulation devices; topical and/or systemic anti-inflammatory medications, such as cyclosporine, diquafosol, and lifitegrast; and surgery.
8
Treatment goals include relieving the signs and symptoms of the disease, improving patients’ comfort, re-establishing ocular surface homeostasis, and minimizing corneal damage.
7
,
9
Irrespective of the severity grade of DED, over-the-counter (OTC) eye drops, or artificial tear products (ATPs) are the mainstay and first-line treatment for DED providing immediate symptomatic relief.
4
,
10
Artificial Tear Products
ATPs are lubricant eye drops used to treat the dryness and irritation associated with DED. These buffered formulations, with or without preservatives, contain electrolytes, surfactants, and one or more lubricants that may be guar- or cellulose-based derivatives including hydroxypropyl guar (HP guar), glycerin, dextran, polyvinyl alcohol, polyethylene glycol [PEG] 400, propylene glycol [PG], sodium hyaluronate, and polyvinylpyrrolidone to enhance or replace the tear film.
8–12
The numerous commercially available ATPs are primarily hypotonic or isotonic buffered solutions containing other excipients (eg, electrolytes, surfactants, and various types of viscosity agents). Most target to replenish either the aqueous or lipid layer of the tear film and there is no consensus on the therapeutic efficacy of one over the other.
13
The ideal artificial tear is one that can spread uniformly and evenly, minimize friction during blinks, has minimal blurring upon instillation, is safe and convenient to use, and effectively improves the signs and symptoms of dry eye.
12
Further, an ideal ATP should also have the potential to restore deficiencies in both, aqueous and lipid, layers of the tear film to address patient’s dry eye symptoms, regardless of the deficiency (lacrimal or meibomian) within the tear film that may be causing the symptoms.
Here, we describe the constituents, mechanisms of action, and clinical evidence of PEG/PG with HP guar that is formulated with an intelligent delivery system and offers symptomatic relief to consumers/patients across the wide spectrum of DED.
Systane Ultra (PEG/PG with HP GUAR)
Systane Ultra
®
(PEG/PG with HP guar; Alcon, Fort Worth, TX, USA) has been commercially available since 2008. PEG/PG with HP guar is commercially available in several countries across four markets (Asia-Pacific, Europe/Middle East/Africa, Latin America/Caribbean, and North America) worldwide, and provides immediate comfort, extended ocular surface protection, and symptom relief for DED due to insufficient quantity or quality of natural tears.
14
Formulation
The active ingredients of PEG/PG with HP guar lubricant eye drops are the hydrophilic demulcents 0.4% PEG 400 and 0.3% PG, which have lower viscosity than cellulose derivatives.
14
The lubricant drops also contain HP guar, a natural polysaccharide gelling agent, and are buffered with borate and sorbitol. Moreover, these lubricant formulations containing PEG/PG with HP guar are free of preservatives.
HP Guar
The HP guar technology was originally developed for ophthalmic use in contact lens multi-purpose solutions (UNIQUE pH™, Alcon Laboratories, Inc., Fort Worth, Texas, USA). Use of HP guar, a water-soluble natural polysaccharide excipient, in PEG/PG with HP guar lubricant drops increases the viscosity of the eye drop owing to its high molecular weight (1000–5000 kDa).
8
,
12
,
14
Although Systane Ultra and Systane Original have similar HP guar concentrations and 2-hour viscosities (range, 4300–5800 Cps), the pH of Systane Ultra (pH 7.9) is higher than that of Systane Original (pH 7.0). Moreover, sorbitol is included in Systane Ultra but not in Systane Original. Upon exposure to the higher pH (~7.5) of the ocular surface and tears, HP guar’s interaction with borate and divalent ions in the tear film allows the formation of the protective viscoelastic matrix on the ocular surface to provide prolonged retention of the active demulcents, thereby protecting the ocular surface. Further, this gel matrix mimics the mucin in the tear film and reduces the friction between the eyelid and ocular surface during blinks.
8
,
12
,
14
HP guar molecules preferentially bind to desiccated or damaged hydrophobic regions of the cornea; this allows protective layer to limit further damage and time for surface epithelial cells to undergo repair and renewal.
14–16
A preclinical study using in vivo (desiccated corneas of anesthetized rabbits) and in vitro (immortalized human corneal epithelial cells and Chang conjunctival cells) models, based on methylene blue uptake, showed that HP guar, PEG, and PG effectively and uniformly formed a layer on the ocular surface providing protection from desiccation, thereby allowing recovery of the damaged epithelium.
16
In another preclinical in vivo study, the effect of lubricant drops containing PEG/PG with HP guar on precorneal mucous layer was evaluated over 7 days in New Zealand white rabbits (N = 16).
17
Their right eyes were treated with PEG/PG/HP guar and the left eyes were randomized to receive PEG/PG/HP guar with Polyquad, 0.1% hyaluronate sodium, 0.5% carboxymethyl cellulose (CMC), and phosphate-buffered saline. The study showed a significantly thicker mucous in eyes treated with PEG/PG/HP guar drops compared with PEG/PG/HP guar with Polyquad (
P
<0.001 vs all). Interestingly, no significant difference was noted in the mucous layer between the eyes treated with PEG/PG/HP guar and PEG/PG/HP guar with Polyquad.
17
In a clinical study of 87 patients with DED, Christensen et al
15
demonstrated that, compared to control (CMC sodium; Refresh Tears
®
Lubricant Eye Drops, Allergan, Irvine, CA, USA), PEG/PG with HP guar lubricant significantly reduced conjunctival (
P
= 0.025) and temporal corneal staining (
P
= 0.024). Furthermore, patients reported a reduction in DED symptoms in the morning (
P
= 0.015) and evening (
P
= 0.023), lower frequencies of foreign body sensation (
P
= 0.033), and felt their eyes were “refreshed longer” (
P
=0.037); thereby confirming that PEG/PG with HP guar lubricant drops were more effective at alleviating signs and symptoms of dry eye than CMC sodium alone (Refresh Tears).
15
Tear instability and increased evaporation in all types of DED results in tear film hyperosmolarity (one of the key characteristics of the disease). A clinical study by Ng et al
18
in 31 patients with DED (with Ocular Surface Disease Index questionnaire [OSDI] ≥20 and mean tear osmolarity ≥300 mOsm/L in at least one eye) showed that treatment with PEG/PG with HP guar for 3 weeks (4 times/day [QID] for 3 weeks) showed a significant reduction in tear osmolarity scores (mean, standard deviation [SD]: 314.6 [11.9] mOsm/L vs 307.7 [15.7] mOsm/L,
P
<0.05) compared to baseline. Furthermore, significant improvements were observed in dry eye conditions and symptoms at Week 3 (mean OSDI score:
P
≤ 0.01; non-invasive tear break‑up time [NIBUT]:
P
<0.05; central corneal staining:
P
<0.05) from baseline. The study further noted that, unlike other hypotonic drops, the reduction in tear osmolarity was observed even 15 minutes after instillation with PEG/PG with HP guar.
18
In 2015, a pre-clinical study by Rangarajan et al explored a potential synergistic benefit of combining HP guar with another naturally viscoelastic hydrophilic polymer, hyaluronic acid (HA), that has also been shown to reduce ocular surface damage in DED patients.
19
,
20
It was observed that in human corneal epithelial cells, hydration protection against desiccation and protection by surface retention were significantly greater with HA/HP guar vs HP guar or HA alone (
P
<0.001). Also, protection with HP guar alone was significantly greater vs HA alone (
P
=0.016). Post surfactant-insult, cell viability, cell barrier protection, tissue hydration, and lubricity were also significantly greater with dual-polymer formulation than HP guar or HA alone.
20
Overall, these findings confirm that HP guar in lubricant eye drops improves the adherence and retention of the ATP, improves tear stability and tissue hydration, and minimizes tear evaporation, thereby lowering tear osmolarity and corneal dryness to provide long-lasting relief from DED symptoms.
Preservatives in PEG/PG with HP Guar
The treatment options for DED are constantly evolving; however, use of lubricant eye drops is a constant through different stages of the disease to provide symptomatic relief. With the long-term and continuous usage of these lubricant drops, the ATPs should be sterile to prevent unwanted effects on the ocular surface, and hence preservatives are added to ATP formulations for their antimicrobial properties.
21
,
22
Furthermore, considering the long-term and frequent usage of the ATPs, it is important that the preservatives added to the ATPs are well tolerated with minimal or no side effects.
21
The most commonly used preservative in prescription topical ophthalmic drops, including ATPs, is benzalkonium chloride (BAK), which demonstrates pan-antimicrobial properties; however, BAK-containing formulations have shown to have deleterious effects on the ocular surface and are not widely used in OTC ocular lubricants or artificial tears.
21–23
Over the last decade, PEG/PG with HP guar lubricant eye drops have shown to provide relief from the symptoms of dry eye without the use of certain preservatives that have adverse effects. Unlike many OTC eye drops, the preservative used in PEG/PG with HP guar is polyquaternium or Polyquad, a hydrophilic cationic polymer that was initially developed and used as an antimicrobial disinfectant in contact lens solutions. The large molecular size of Polyquad is key to its function, while it effectively disrupts microbial membranes, its sheer size exclusion prevents cytotoxicity in mammalian cells.
21
,
24
Polyquad has shown to be effective against
Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus
, and the fungus
Candida albicans
.
25
With more than 30 years of usage, safety concerns raised with Polyquad are minimal – it does not adversely affect the ocular surface and has shown to offer comparable tolerability to a preservative-free eye drops.
17
,
21
,
22
The concerns over the use of preservatives in ATPs, in addition to physician and patient preferences, have led to the development of preservative-free formulations. While preservative-based eye drops have a longer shelf life, preservative-free drops typically come in disposable single-dose sterile vials that reduce the risk of contamination and eliminate the need for preservatives. PEG/PG with HP guar preservative-free lubricant eye drops relieve dry eye symptoms with a preservative-free formula and are available in convenient, single-use sterile vials that provide immediate and long-lasting DED symptom relief similar to its preserved formulation.
17
Lubricant eye drops are now also available in multi-dose preservative-free formulations that are cost effective and easy to use.
21
,
22
,
26
Mechanism of Action
PEG/PG with HP guar lubricant eye drops are maintained at a pH of 7.9 where HP guar, borate, and sorbitol exist in a state of dynamic equilibrium where sorbitol optimizes the viscosity of the drop.
12
During instillation, the pressure exerted on the bottle reduces the drop viscosity and once instilled, the concentration of sorbitol decreases owing to its water solubility, allowing for efficient and uniform spreading. The borate ions in Systane interact with galactomannan on the surface of the eye to form a protective cross-linked bio-adhesive gel.
12
,
14
,
27
Further, the ionic properties of the tear film increase the density of the borate/HP guar crosslinks and fortify this low viscosity gel matrix to allow retention of active demulcents for tear film stability and lubrication, decrease evaporation of the tear film, and protect the ocular surface (
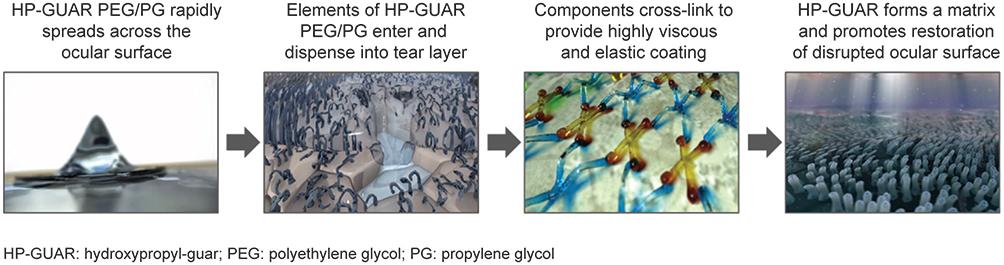
Figure 1
). The borate/HP guar gel matrix also reduces friction between blinks to provide prolonged relief and comfort.
14
Figure 1
Mechanism of action of PEG/PG with HP GUAR. Image courtesy with permission from Alcon.
Abbreviations:
HP GUAR, hydroxypropyl-guar; PEG, polyethylene glycol; PG, propylene glycol.
The aforementioned properties make PEG/PG with HP guar an ideal lubricating drop with a longer retention time and sustained lubrication for aqueous-deficient, evaporative, and mixed DED conditions.
Clinical Studies of PEG/PG with HP Guar
A literature search was conducted independently by the authors on the PubMed database; following removal of duplicates, a total of 304 articles were identified using the following search strings: (i) (Systane Ultra) AND (dry eye disease OR DED OR dry eye syndrome OR DES or dry eye); (ii) ([polyethylene glycol OR PEG] AND [propylene glycol OR PG] AND [hydroxypropyl guar OR HP guar OR HPG]) AND (dry eye disease OR DED OR dry eye syndrome OR DES or dry eye); (iii) (Systane Ultra) AND (artificial tears OR ocular lubricants OR lubricant eye drops OR ATP); (iv) ([polyethylene glycol OR PEG] AND [propylene glycol OR PG] AND [hydroxypropyl guar OR HP guar OR HPG]) AND (artificial tears OR ocular lubricants OR lubricant eye drops OR ATP); and (v) (dry eye OR DED) AND (artificial tears AND lubricant). Multiple reports of the same dataset were assessed, and only the most updated articles were included. Only English language articles were included. Abstracts retrieved from the search were screened and a total of 20 relevant articles were identified, with consensus, by the authors were further evaluated and reviewed. In addition, 3 relevant abstracts on the use of PEG/PG with HP guar in DED identified from Google Scholar Search were also identified and reviewed. Data from the studies reporting on the use of PEG/PG with HP guar in dry eye conditions, including the study design, patient population, lubricant dosage, and endpoints assessed are summarized in
Figure 2
.
Figure 2
Flow chart for literature search for publication screening and inclusion. *Search strings used: (i) (Systane Ultra) AND (dry eye disease OR DED OR dry eye syndrome OR DES or dry eye); (ii) ([polyethylene glycol OR PEG] AND [propylene glycol OR PG] AND [hydroxypropyl guar OR HP guar OR HPG]) AND (dry eye disease OR DED OR dry eye syndrome OR DES or dry eye); (iii) (Systane Ultra) AND (artificial tears OR ocular lubricants OR lubricant eye drops OR ATP); (iv) ([polyethylene glycol OR PEG] AND [propylene glycol OR PG] AND [hydroxypropyl guar OR HP guar OR HPG]) AND (artificial tears OR ocular lubricants OR lubricant eye drops OR ATP); and (v) (dry eye OR DED) AND (artificial tears AND lubricant).
†
After removing duplicates.
Over the last 10 years, clinical studies have demonstrated that the use of PEG/PG with HP guar is associated with improvements in the signs and symptoms of dry eye and is well tolerated in patients with DED (
Table 1
). Here, we present an overview of key clinical studies conducted with PEG/PG with HP guar in patients with dry eye.
Table 1
Clinical Studies Using PEG/PG with HP Guar Lubricant Eye Drops in DED
Efficacy and Safety of PEG/PG with HP Guar (Systane Ultra)
A 6-week, controlled, randomized, prospective, double-masked, multisite, parallel-group study conducted by Davitt et al
28
on 113 patients with DED evaluated the efficacy and safety of PEG/PG with HP guar (vs saline) in improving the signs and symptoms of DED. The study demonstrated a reduction in the severity of dry eye with no untoward safety issues.
28
Use of PEG/PG with HP guar lubricant eye drops reduced both corneal and conjunctival staining as early as Day 7, with significant reduction in mean corneal staining (
P
= 0.0009) by Day 14 and in mean conjunctival staining (
P
= 0.0475) by Day 28 that was sustained until end of the study. Furthermore, with PEG/PG with HP guar use patients also reported significant reductions in the mean scores for the ocular symptoms of dryness and OSDI vs baseline. The authors concluded that PEG/PG with HP guar lubricant was efficacious and well tolerated when used QID for 6 weeks in patients with dry eyes.
28
The efficacy of ATPs in relieving symptoms of dry eye typically rely on patient reporting, which may be subjective.
29
Therefore, in addition to patient-reported scores, clinicians evaluate signs of ocular damage by staining for specific ocular surface antigens that are altered during DED. Human leukocyte antigen-
DR
isotype (HLA-DR) is one such biomarker for increased inflammation on the ocular surface associated with DED.
30
,
31
Fernandez et al
32
showed that treatment with PEG/PG with HP guar for 30 days significantly reduced HLA-DR expression (
P
= 0.02), corneal staining (
P
= 0.01), OSDI score (
P
= 0.02), and tear firm breakup time (TFBUT;
P
= 0.01), suggesting a reduction in surface inflammation and thereby an improvement in the signs and symptoms of DED.
In 2017, Labetoulle et al
33
demonstrated that PEG/PG with HP guar decreased ocular surface staining based on the mean change in total ocular surface staining (TOSS) score from baseline at Day 35 (mean [SD]: −2.2 [0.33] points), thereby alleviating the signs and symptoms in patients with DED. Further, patient-reported scores from the study indicated that DED treatment with PEG/PG with HP guar was effective, convenient, and well tolerated over 3 months of treatment.
33
Asbell et al
34
showed that PEG/PG with HP guar eye drops instilled QID or PRN to DED patients (N = 97) reduced corneal staining but the difference between the two dosing regimens for reduction in TOSS score was not statistically significant (mean [SD]: −1.19 [0.26] for QID vs −0.94 [0.24] for PRN;
P
= 0.184). However, the Impact of Dry Eye on Everyday Life (IDEEL) symptom-bother score favored QID dosing, suggesting that the regular use of artificial tears may provide improved symptom relief vs PRN use in DED. Overall, both regimens were well tolerated with no new safety findings.
34
As mentioned earlier, Ng et al showed a significant (
P
<0.05) reduction in osmolarity (15 minutes after instillation), in addition to improvements in dry eye symptoms (conjunctival hyperemia, ocular surface staining, and central corneal staining) using PEG/PG with HP guar QID over 3 weeks in DED patients (N = 31).
18
More recently, a study by Aguilar et al
35
demonstrated the efficacy of PEG/PG with HP guar (thrice daily) at Day 90 (vs baseline) in reducing squamous metaplasia (based on mean ± SD goblet cell density score: 0.8 ± 0.5 vs 1.2 ± 0.5;
P
<0.0001), improving TFBUT (6.8 vs 5.8;
P
<0.0001), corneal staining (0.3 vs 3.1; P <0.00010), and conjunctival staining (0.9 vs 3.6; P <0.0001), thereby allowing normalization of the ocular surface in patients with DED.
Comparator Studies: PEG/PG with HP Guar vs Other ATPs/Treatments
With more than a decade of effectively alleviating the signs and symptoms of dry eye without any major safety concerns, PEG/PG with HP guar is frequently used as a standard/comparator while assessing the effectiveness of other lubricant eye drops in DED.
PEG/PG with HP Guar vs Optive
PEG/PG with HP guar has been compared with Optive
®
Tears (CMC and glycerin; Allergan;
https://www.allergan.com.au/en-au/products/list/optive-lubricant-eye-drops-15mL
, last accessed 07-February 2020) across four different studies.
28
,
33
,
36
,
37
Davitt et al
28
were the first to compare the efficacy and safety of PEG/PG with HP guar with that of Optive in adult patients with dry eye (N = 105). No differences were observed between the two products with respect to symptomatic relief (treatment satisfaction, post-treatment ocular symptom scores, or OSDI outcomes). However, patients treated with PEG/PG with HP guar had significantly lower mean corneal staining at Days 14 (
P
= 0.0009) and 42 (
P
= 0.0106) and significant reductions in conjunctival staining at Days 28 (
P
= 0.0475) and 42 (
P
= 0.0009).
28
A more recent study by Labetoulle et al
33
demonstrated that PEG/PG with HP guar was non-inferior to Optive based on mean change in TOSS score from baseline to Day 35 (mean ± SD: −2.2 ± 0.33 [PEG/PG with HP guar] vs −1.7 ± 0.47 [Optive];
P
= 0.38). Both treatments improved ocular surface health, alleviated signs and symptoms of dry eye, and had similar patient-reported scores for treatment effectiveness (62.2 [PEG/PG with HP guar] vs 55.7 [Optive]) and inconvenience (69.5 [PEG/PG with HP guar] vs 67.1 [Optive]).
33
Two independent studies evaluated the effect of PEG/PG with HP guar vs Optive on visual function. The first, conducted in patients with DED (N = 48), showed that PEG/PG with HP guar improved maintenance of visual acuity (VA; as measured by the Interval Visual Acuity Decay [IVAD] test) between blinks at 90 minutes post-instillation vs Optive (
P
= 0.0365).
36
Overall, both PEG/PG with HP guar and Optive were well tolerated, with no significant differences between mean scores reading rate (
P
= 0.38) and functional blink rate (
P
= 0.15).
36
A study by Guillon et al
37
compared the effect of PEG/PG with HP guar vs Optive on visual performance. The results showed that, in patients with mild-to-moderate DED (N = 54), both PEG/PG with HP guar and Optive provided significant improvements in patient-reported vision and comfort with relieved symptoms (
P
<0.001). Furthermore, repeated usage of PEG/PG with HP guar provided beneficial effects with respect to visual performance.
37
PEG/PG with HP Guar vs Rohto
Thus far, two studies have compared PEG/PG with HP guar with Rohto
®
(The Mentholatum Company, Orchard Park, NY, USA,
http://www.rohtoeyedrops.com/professionals/
, last accessed 07 February, 2020) lubricant drops, where PEG/PG with HP guar was used as a comparator.
38
,
39
Rohto is a sterile, buffered product packaged in a multi-dose container containing propylene glycol and povidone in a clear microemulsion formulation. In a prospective, double-blind, crossover study in normal and DED patients, Corcoran et al
39
showed that mean corneal sensitivity scores were equivalent and normal in both patient groups at baseline and 10 min after instillation of Rohto Hydra and PEG/PG with HP guar (
P
= 0.24). Mean cooling scores post Rohto Hydra and PEG/PG with HP guar instillation was higher in DED patients vs normal at all assessed time points. While sum cooling scores with both lubricant drops were significantly higher in DED patients vs normal, cooling response with Rohto Hydra was greater vs PEG/PG with HP guar only in the DED group (DED:
P
= 0.004; normal:
P
= 0.320).
39
Torkildsen et al
38
showed that both lubricant drops (administered twice-a-day) were comparable in improving the signs and symptoms of DED over the 4-week assessment period based on ocular staining, tear film metrics, ocular comfort (

P
= 0.364), and visual function assessments. Based on the quality of life questionnaire, improvements in three of four metrics, including driving, were greater for the Rohto users, but the differences were not statistically significant.
38
PEG/PG with HP Guar vs Blink Tears
In patients with mild-to-moderate DED (N =40), Blink Tears
®
(polyethylene glycol [PEG], Johnson & Johnson,
https://www.justblink.com/find-your-products/dry-eye-lubricating-eye-drops/blink-tears-lubricating-eye-drops?upcean=329943002156
, last accessed 07 February, 2020) significantly improved TBUT after 1 month of treatment (
P
= 0.003) and no significant changes were noted for staining or VA. Patients reported that Blink Tears was more soothing, improved vision, resulted in less blurring, and was more comfortable than PEG/PG with HP guar (
P
<0.043).
40
PEG/PG with HP Guar vs Refresh
Thus far, two studies have compared the efficacy of PEG/PG with HP guar and Refresh tears (CMC sodium).
41
,
42
In 2012, Waduthantri et al
41
compared the efficacy of Refresh vs PEG/PG with HP guar in Chinese patients with DED (N =30). They demonstrated that there was no significant difference in the efficacy of these drugs in terms of improving symptoms and altering objective signs of dry eye, such as corneal fluorescein staining, TBUT, and Schirmer’s test results (
P
>0.05).
41
In an in vitro comparative toxicity study of preservative vs preservative-free ATPs, preservative-free formulations were less cytotoxic to cultured corneal epithelial cells vs their preservative-containing counterparts. While ATPs with preservatives including PEG/PG with HP guar, Blink, and GenTeal
®
(hydroxypropyl methylcellulose 0.3%; Novartis, Novartis [India] Ltd) demonstrated the most in vitro cytotoxicity, non-preserved Refresh Preservative-Free lubricant drops were least cytotoxic to cultured corneal epithelial cells.
42
PEG/PG with HP Guar vs Optimel
Wong et al
43
demonstrated that in 24 patients with contact lens-related DED, treatment with both PEG/PG with HP guar and Optimel
®
(Manuka honey; Melcare Biomedical Pty Lt, Queensland, Australia) improved DED symptoms. However, comparison of the two lubricant drops demonstrated treatment differences between Optimel and PEG/PG with HP guar based on OSDI (
P
= 0.02) and ocular comfort index (
P
= 0.05) scores.
43
PEG/PG with HP Guar vs Genteal Eye Drops
A retrospective analysis was conducted by Maharana et al
44
on patients with DED to compare the efficacy of CMC (4X day), PEG/PG with HP guar (2X day), and Genteal Eye Drops. The results demonstrated that patients treated with PEG/PG with HP guar demonstrated significant improvements in mean OSDI (
P
= 0.0), TBUT (
P
≤ 0.01), and in Schirmer's test (
P
≤ 0.02) vs those treated with CMC at all assessed follow-up time points (0–1, 0–4, and 1–4 weeks). No significant difference was noted for OSDI, TBUT, and Schirmer’s test assessments between patients treated with PEG/PG with HP guar and those treated with HPMC 0.3%.
44
PEG/PG with HP Guar is Well Tolerated in Contact Lens Wearers
Contact lens wear is often associated with symptoms of ocular irritation, including symptoms of dryness, discomfort, soreness, and tiredness. Among contact lens wearers, 25% to 50% suffer from contact lens-related dry eye.
45
Kading et al
46
provided evidence of the safety and compatibility of PEG/PG with HP guar lubricant eye drops in contact lens wearers. They showed that PEG/PG with HP guar usage in contact lens wearers was not associated with any significant change in corneal staining, distance VA, or with any adverse events.
46
In 2014, McDonald et al
45
assessed the clinical performance of PEG/PG with HP guar in daily disposable soft contact lens wearers. The study demonstrated that the use of PEG/PG with HP guar over 2 weeks resulted in a significant increase in comfortable lens wear time (
P
= 0.007). A significant reduction in overall dryness (
P
<0.0001) and end-of-the day dryness (
P
= 0.047) were also observed PEG/PG with HP guar.
45
Overall, these studies suggest that PEG/PG with HP guar provide extended protection and provide long-lasting relief from dryness symptoms associated with contact lens usage.
PEG/PG with HP Guar Usage Under Environmental Conditions and During/Post-Cataract Surgery
Adverse environmental conditions worsen the signs and symptoms of dry eye. Differences in temperature and humidity may affect the efficacy of ATPs in alleviating DED symptoms.
3
,
4
In patients with mild-to-moderate dry eye symptoms (N = 30), Gokul et al
47
showed that a single application of PEG/PG with HP guar had protective effects against exposure to dry conditions. Following exposure to a validated simulated adverse environment model created by a 45 cm 55-W standing fan directed towards the eye, at a distance of 1m for 2.5 minutes, PEG/PG with HP guar increased NIBUT (
P
<0.001) and prevented its decline below baseline.
47
While the manufacturer’s recommendation is to store PEG/PG with HP guar at room temperature, Bitton et al
48
demonstrated that the effect of PEG/PG with HP guar in providing comfort is the same irrespective of storage temperature. Based on patient-reported outcomes, the study did not reveal any advantage in refrigerating the drops prior to use either in the morning or late in the day in mild-to-moderate DED patients.
48
In a prospective, parallel, randomized, investigator-masked, single-center, clinical study, Davidson et al
49
evaluated the efficacy of PEG/PG with HP guar in patients with a history of episodic eye irritation or dryness related to environmental factors seeking routine cataract extraction. They showed that the use of PEG/PG with HP guar (in addition to standard of care) provided ocular surface protection and significant improvements in ocular surface comfort, as well as signs and symptoms of dry eye. Based on these results, the authors suggested that PEG/PG with HP guar might contribute to improvement in post-cataract surgical outcomes.
49
Further, a comparative study with PEG/PG with HP guar and Hylocomod (Farmex, Greece) in patients who underwent cataract extraction surgery showed that both ATPs provided a significant improvement in TBUT index (
P
<0.05) associated with a reduction in foreign body sensation through the postoperative period and a significant reduction of the blinking discomfort for the first postoperative week.
50
Overall, the study demonstrated that both PEG/PG with HP guar and Hylocomod were equally efficient in alleviating ocular surface disease symptoms following cataract extraction surgery.
50
Overall, these clinical studies demonstrate the clinical efficacy and safety of PEG/PG with HP guar in improving the signs of DED while providing lasting relief from symptoms. The interaction between HP guar, borate, and divalent ions in the tear film results in the formation of viscoelastic matrix that allows longer retention of active demulcents and protects ocular surface.
8
,
12
,
14
HP guar molecules form a protective layer around the compromised hydrophobic areas of cornea, which avoids further damage and allows time for repair and lubrication of the surface. Furthermore, the addition of Polyquad, a hydrophilic cationic polymer, prevents accidental contamination and shown to be effective against
Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus
, and the fungus
Candida albicans
.
25
After more than three decades of usage, the Polyquad is neither associated with any safety concerns nor has adverse effect on the ocular surface.
17
,
21
,
22
Although these unique properties of demulcents and safety and efficacy profile of PEG/PG with HP guar are reported by single arm or comparator studies, it is important to note that this literature review is purely descriptive in nature and we do not intend to draw comparisons with other products. Inter-study differences, including study design, populations, treatment regimens, follow-up period, or statistical methods used for data analysis in publications, may account for the variability observed in the endpoints assessed across these studies. Therefore, comparison of data between different studies may be inconclusive and such be interpreted with caution.
Summary
The presence of lower viscosity demulcents and other excipients (including HP guar and sorbitol) allows PEG/PG with HP guar to form a viscoelastic layer of protection to heal damaged areas of the cornea and to provide extended ocular surface protection and symptom relief. Inclusion of sorbitol PEG/PG with HP guar minimizes blurring. Overall, PEG/PG with HP guar effectively alleviates signs and symptoms of dry eye, including conjunctival and cornea surface staining as well as patient-reported scores for treatment effectiveness and inconvenience. PEG/PG with HP guar has been shown to improve DED symptoms associated with cataract surgery, environmental conditions, and contact lens usage. Clinically, PEG/PG with HP guar lubricant eye drops have been shown to enhance tear film stability, visual function, comfort with contact lens wear, and improved VA in dry eye patients. With over a decade of usage, PEG/PG with HP guar is effective, convenient to use, and well tolerated in DED.
Disclosure
S.S. and V.M. are both employees of Alcon Research, LLC, Johns Creek, Georgia, USA. The authors report no other conflicts of interest in this work.
References
1.
Moon JH, Kim KW, Moon NJ. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study.
BMC Ophthalmol
. 2016;16(1):188. doi:10.1186/s12886-016-0364-4
2.
International Dry Eye WorkShop Study Group. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007).
Ocul Surf
. 2007;5(2):93–107. doi:10.1016/S1542-0124(12)70082-4
3.
Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary.
Ocul Surf
. 2017;15(4):802–812. doi:10.1016/j.jtos.2017.08.003
4.
Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R. TFOS DEWS II management and therapy report.
Ocul Surf
. 2017;15(3):575–628.
5.
Patel VD, Watanabe JH, Strauss JA, Dubey AT. Work productivity loss in patients with dry eye disease: an online survey.
Curr Med Res Opin
. 2011;27(5):1041–1048. doi:10.1185/03007995.2011.566264
6.
O’Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies.
Curr Allergy Asthma Rep
. 2004;4(4):314–319. doi:10.1007/s11882-004-0077-2
7.
Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease.
Dtsch Arztebl Int
. 2015;112(5):71–81. doi:10.3238/arztebl.2015.0071
8.
Nassiri N, Rodriguez Torres Y, Meyer Z, Beyer MA, Vellaichamy G, Dhaliwal AS. Current and emerging therapy of dry eye disease. Part A: pharmacological modalities.
Exp Rev Ophthalmol
. 2017;12(4):269–297. doi:10.1080/17469899.2017.1327350
9.
Sutu C, Fukuoka H, Afshari NA. Mechanisms and management of dry eye in cataract surgery patients.
Curr Opin Ophthalmol
. 2016;27(1):24–30. doi:10.1097/ICU.0000000000000227
10.
Gayton JL. Etiology, prevalence, and treatment of dry eye disease.
Clin Ophthalmol
. 2009;3:405–412. doi:10.2147/OPTH.S5555
11.
Che Arif FA, Hilmi MR, Kamal KM, Ithnin MH. Evaluation of 18 artificial tears based on viscosity and pH.
Malaysian J Ophthalmol
. 2020;2(2):96–111. doi:10.35119/myjo.v2i2.109
12.
Springs C. Novel ocular lubricant containing an intelligent delivery system: details of its mechanism of action.
Dev Ophthalmol
. 2010;45:139–147.
13.
Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome.
Cochrane Database Syst Rev
. 2016;2:CD009729. doi:10.1002/14651858.CD009729.pub2
14.
Benelli U. Systane lubricant eye drops in the management of ocular dryness.
Clin Ophthalmol
. 2011;5:783–790. doi:10.2147/OPTH.S13773
15.
Christensen MT, Cohen S, Rinehart J, et al. Clinical evaluation of an HP guar gellable lubricant eye drop for the relief of dryness of the eye.
Curr Eye Res
. 2004;28(1):55–62. doi:10.1076/ceyr.28.1.55.23495
16.
Ubels JL, Clousing DP, Van Haitsma TA, et al. Pre-clinical investigation of the efficacy of an artificial tear solution containing hydroxypropyl-guar as a gelling agent.
Curr Eye Res
. 2004;28(6):437–444. doi:10.1080/02713680490503787
17.
Moon SW, Hwang JH, Chung SH, Nam KH. The impact of artificial tears containing hydroxypropyl guar on mucous layer.
Cornea
. 2010;29(12):1430–1435. doi:10.1097/ICO.0b013e3181ca636b
18.
Ng A, Keech A, Jones L. Tear osmolarity changes after use of hydroxypropyl-guar-based lubricating eye drops.
Clin Ophthalmol
. 2018;12:695–700. doi:10.2147/OPTH.S150587
19.
Aragona P, Papa V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye.
Br J Ophthalmol
. 2002;86(2):181–184. doi:10.1136/bjo.86.2.181
20.
Rangarajan R, Kraybill B, Ogundele A, Ketelson HA. Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium.
J Ocul Pharmacol Ther
. 2015;31(8):491–497. doi:10.1089/jop.2014.0164
21.
Walsh K, Jones L. The use of preservatives in dry eye drops.
Clin Ophthalmol
. 2019;13:1409–1425. doi:10.2147/OPTH.S211611
22.
Moshirfar M, Pierson K, Hanamaikai K, Santiago-Caban L, Muthappan V, Passi SF. Artificial tears potpourri: a literature review.
Clin Ophthalmol
. 2014;8:1419–1433. doi:10.2147/OPTH.S65263
23.
Charnock C. Are multidose over-the-counter artificial tears adequately preserved?
Cornea
. 2006;25(4):432–437. doi:10.1097/01.ico.0000183538.53017.69
24.
Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells.
Adv Ther
. 2010;27(11):837–845. doi:10.1007/s12325-010-0070-1
25.
Codling CE, Maillard JY, Russell AD. Aspects of the antimicrobial mechanisms of action of a polyquaternium and an amidoamine.
J Antimicrob Chemother
. 2003;51(5):1153–1158. doi:10.1093/jac/dkg228
26.
Ribeiro M, Barbosa FT, Ribeiro LEF, Sousa-Rodrigues CF, Ribeiro EAN. Effectiveness of using preservative-free artificial tears versus preserved lubricants for the treatment of dry eyes: a systematic review.
Arq Bras Oftalmol
. 2019;82(5):436–445. doi:10.5935/0004-2749.20190097
27.
Meadows DL, Ketelson HA, Davis J. Extensional rheological properties of an artificial tear delivery system.
Invest Ophthalmol Vis Sci
. 2008;49(13):1545.
28.
Davitt WF, Bloomenstein M, Christensen M, Martin AE. Efficacy in patients with dry eye after treatment with a new lubricant eye drop formulation.
J Ocul Pharmacol Ther
. 2010;26(4):347–353. doi:10.1089/jop.2010.0025
29.
Bhatnagar KR, Pote S, Pujari S, Deka D. Validity of subjective assessment as screening tool for dry eye disease and its association with clinical tests.
Int J Ophthalmol
. 2015;8(1):174–181. doi:10.3980/j.issn.2222-3959.2015.01.31
30.
Tsubota K, Fujihara T, Saito K, Takeuchi T. Conjunctival epithelium expression of HLA-DR in dry eye patients.
Ophthalmologica
. 1999;213(1):16–19. doi:10.1159/000027387
31.
Versura P, Profazio V, Schiavi C, Campos EC. Hyperosmolar stress upregulates HLA-DR expression in human conjunctival epithelium in dry eye patients and in vitro models.
Invest Ophthalmol Vis Sci
. 2011;52(8):5488–5496. doi:10.1167/iovs.11-7215
32.
Fernandez KB, Epstein SP, Raynor GS, et al. Modulation of HLA-DR in dry eye patients following 30 days of treatment with a lubricant eyedrop solution.
Clin Ophthalmol
. 2015;9:1137–1145. doi:10.2147/OPTH.S81355
33.
Labetoulle M, Messmer EM, Pisella PJ, Ogundele A, Baudouin C. Safety and efficacy of a hydroxypropyl guar/polyethylene glycol/propylene glycol-based lubricant eye-drop in patients with dry eye.
Br J Ophthalmol
. 2017;101(4):487–492. doi:10.1136/bjophthalmol-2016-308608
34.
Asbell P, Vingrys AJ, Tan J, Ogundele A, Downie LE. Clinical outcomes of fixed versus as-needed use of artificial tears in dry eye disease: a 6-week, observer-masked Phase 4 clinical trial.
Invest Ophthalmol Vis Sci
. 2018;59(6):2275–2280. doi:10.1167/iovs.17-23733
35.
Aguilar A, Berra M, Tredicce J, Berra A. Efficacy of polyethylene glycol-propylene glycol-based lubricant eye drops in reducing squamous metaplasia in patients with dry eye disease.
Clin Ophthalmol
. 2018;12:1237–1243. doi:10.2147/OPTH.S164888
36.
Torkildsen G. The effects of lubricant eye drops on visual function as measured by the Inter-blink interval Visual Acuity Decay test.
Clin Ophthalmol
. 2009;3:501–506. doi:10.2147/OPTH.S6225
37.
Guillon M, Maissa CA, Wong S, Griffin JM, Christensen MT. Functional visual performance of systane ultra after 4 weeks of use.
Invest Ophthalmol Vis Sci
. 2011;52(14):3834.
38.
Torkildsen G, Brujic M, Cooper MS, Karpecki P, Majmudar P. Evaluation of a new artificial tear formulation for the management of tear film stability and visual function in patients with dry eye.
Clin Ophthalmol
. 2017;11:1883–1889. doi:10.2147/OPTH.S144369
39.
Corcoran P, Hollander DA, Ousler GW, et al. Dynamic sensitivity of corneal TRPM8 receptors to menthol instillation in dry eye versus normal subjects.
J Ocul Pharmacol Ther
. 2017;33(9):686–692. doi:10.1089/jop.2017.0050
40.
Kislan T. Cross-over evaluation of blink tears versus systane ultra in mild dry eye patients.
Optometry
. 2010;81(6):287. doi:10.1016/j.optm.2010.04.041
41.
Waduthantri S, Yong SS, Tan CH, Htoon HM, Tong L. Lubricant with gelling agent in treating dry eye in adult Chinese patients.
Optom Vis Sci
. 2012;89(11):1647–1653. doi:10.1097/OPX.0b013e31826cfc41
42.
Zheng LL, Myung D, Yu CQ, Ta CN. Comparative In vitro cytotoxicity of artificial tears.
JSM Ophthalmol
. 2015;3(1):1026.
43.
Wong D, Albietz JM, Tran H, Du Toit C, Li AH. Treatment of contact lens related dry eye with antibacterial honey.
Cont Lens Anterior Eye
. 2017;40(6):389–393. doi:10.1016/j.clae.2017.10.001
44.
Maharana PK, Raghuwanshi S, Chauhan AK, Rai VG, Pattebahadur R. Comparison of the efficacy of carboxymethylcellulose 0.5%, hydroxypropyl-guar containing polyethylene glycol 400/propylene glycol, and hydroxypropyl methyl cellulose 0.3% tear substitutes in improving ocular surface disease index in cases of dry eye.
Middle East Afr J Ophthalmol
. 2017;24(4):202–206. doi:10.4103/meajo.MEAJO_165_15
45.
McDonald M, Schachet JL, Lievens CW, Kern JR. Systane(R) ultra lubricant eye drops for treatment of contact lens-related dryness.
Eye Contact Lens
. 2014;40(2):106–110. doi:10.1097/ICL.0000000000000018
46.
Kading D. A two-week clinical evaluation of the safety of systane ultra in contact lens-wearing patients.
Clin Ophthalmol
. 2010;4:27–32. doi:10.2147/OPTH.S8079
47.
Gokul A, Wang MTM, Craig JP. Tear lipid supplement prophylaxis against dry eye in adverse environments.
Cont Lens Anterior Eye
. 2018;41(1):97–100. doi:10.1016/j.clae.2017.09.013
48.
Bitton E, Crncich V, Brunet N. Does the temperature of an artificial tear affect its comfort?
Clin Exp Optom
. 2019;101(5):641–647. doi:10.1111/cxo.12664
49.
Davidson R, Schultz-Scott B. Efficacy evaluation of systane
®
ultra in patients with dry eye undergoing cataract surgery. In:
30th Congress of the European Society of Cataract and Refractive Surgery
. 2012.
50.
Labiris G, Ntonti P, Sideroudi H, Kozobolis V. Impact of polyethylene glycol 400/propylene glycol/hydroxypropyl-guar and 0.1% sodium hyaluronate on postoperative discomfort following cataract extraction surgery: a comparative study.
Eye Vis
. 2017;4:13. doi:10.1186/s40662-017-0079-5
This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at
https://www.dovepress.com/terms.php
and incorporate the
Creative Commons Attribution - Non Commercial (unported, v3.0) License
.By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of
our Terms
.
Download Article
[PDF]
